TYPE: Research Article![]()
Mitigating linear infrastructure: Artificial canopy bridges as a key mitigating tool to reduce golden langur (Trachypithecus geei) road accidents in Assam, India
Jihosuo Biswas¹,²*![]() , Joydeep Shil¹,²*
, Joydeep Shil¹,²*![]() , Kanmaina Ray¹,², Mehtab Uddin Ahmed¹,², Dharma Kanta Ray¹,², Amulya Boro¹,², Puja Muchahary¹,², Benjamin Dorsey³, Honnavalli N Kumara⁴
, Kanmaina Ray¹,², Mehtab Uddin Ahmed¹,², Dharma Kanta Ray¹,², Amulya Boro¹,², Puja Muchahary¹,², Benjamin Dorsey³, Honnavalli N Kumara⁴
¹Primate Research Centre Northeast India, Guwahati-781012, Assam, India.
²Conservation Himalayas Northeastern Chapter, Pandu, Guwahati-781012, Assam, India.
³Asian Development Bank, PO Box- 3145, Revelstoke BC, Canada.
⁴Salim Ali Centre for Ornithology and Natural History, South India Centre of Wildlife Institute of India, Coimbatore, Tamil Nadu, India.
RECEIVED 27 August 2025
ACCEPTED 29 September 2025
ONLINE EARLY 18 November 2025
PUBLISHED 22 December 2025
Abstract
Linear infrastructures like roads and power lines fragment the forest habitats used by golden langurs (Trachypithecus geei) in Assam, India. Artificial gaps in the forest canopy force these arboreal primates to descend to the ground, resulting in roadkill and other forms of anthropogenic mortality. From January 2023 to December 2024, a study in the Chakrashila–Amguri–Buxamara–Nayekgaon forest complex recorded 18 langur-vehicle collisions in the Nayekgaon-Choibari section of the State Highway- 14 (SH-14), leading to seven deaths, five major injuries, and six minor injuries. To mitigate these risks, 15 artificial canopy bridges of four designs were installed along SH-14. During the monitoring, 112 instances of road and canopy bridge crossings by eight golden langur groups were recorded. Langurs used canopy bridges (74%, n = 83) significantly more than the road (χ² = 26.04, df = 1, p < 0.01). Among canopy bridges, pipe (69.9%) and ladder bridges (26.5%) were most effective, reducing ground-level crossings and probable collisions by ~74% during the study. Some power lines in the study area were insulated, providing additional pathways for their movement across the road. The initiative also integrates community outreach education to promote the conservation of golden langurs, providing incentives for restoring corridors through plantations, and maintaining them to mitigate conflicts. These interventions can restore fragmented habitat and, thus, corridor connectivity, reduce mortality risk, and are expected to enhance their persistence in the fragmented landscape.
Keywords: Colobine, collisions, corridor, fragmentation, habitat loss, population sustainability.
Introduction
Arboreal primates depend on undisturbed continuous canopies for their movement and dispersal within their habitats. However, canopy breakage and qualitative degradation of forest habitat by linear infrastructure – such as roads, power lines, and railway lines- severely disrupt their natural movement (Asensio et al., 2021). Even within large forest patches, such breakages can isolate primate groups, and force animals to descend and adopt terrestrial movement to navigate across disconnected areas within their home ranges, thereby increasing the risk of road collisions, electrocution, and exposure to predators (Biswas, 2002; Lokschin et al., 2007; Das et al., 2009; Mass et al., 2011; Donaldson & Cunneyworth, 2015). Lack of canopy connectivity can bring changes in diet (Onderdonk & Chapman, 2000; Das et al., 2009), modifications in home ranges (Onderdonk & Chapman, 2000; Bicca-Marques, 2003; Shil et al., 2021), increased physiological stress and higher parasite loads (Chapman et al., 2006), and greater exposure to predators. Importantly, fragmentation restricts animal movement and gene flow between populations, which may further threaten the long-term viability of species (Biswas, 2002; Mass et al., 2011). According to the IUCN Threats Classification Scheme (Version 3.2), 19.4% of all primate species are threatened by roads and railroads (Praill et al., 2023). The rapid expansion of road networks – especially in and around primate habitat, including remote forested areas that serve as critical refuges – has exacerbated this threat. Consequently, wildlife-vehicle collisions (WVCs) involving primates are becoming increasingly common (Figure 1). Although many primates are primarily arboreal, roads often act as barriers that disrupt their natural movement.

Figure 1: Report of the yearly number of primate road kills in the Global Primate Roadkill Database (GPRD), showing a gradual increase during 1987-2024 (bars), and an acceleration of records in the 2010s. The secondary axis provides the cumulative number of roadkill incidents (line). Data used are availed from The Global Primate Roadkill Database compiled by Praill et al. (2023) (accessed at: https://gprd.mystrikingly.com on 7 August 2025).
The golden langur (Trachypithecus geei) is an obligate canopy-dwelling primate endemic to the Indo-Bhutan border. It is found primarily in four districts of western Assam, India, and six districts of south-central Bhutan, making it one of the most ‘range-restricted’ primate species in South Asia (Biswas et al., 2024; Thinley et al., 2019). Across its range, the species faces habitat loss and population decline, with over half of its natural habitat lost in recent decades (Srivastava et al., 2001). This decline is especially critical in the southern part of their range-particularly in Kokrajhar and Bongaigaon districts- where deforestation and the conversion of forests into agricultural fields and human settlements have fragmented once-continuous forests into smaller, isolated patches, jeopardising the long-term viability of these populations.
Recent population estimates suggest that the fragmented forests of Assam in its southern range support ~25% of India’s golden langur population (Biswas et al., 2024). However, several golden langur sub-populations of them have suffered a drastic decline or local extinctions in recent years due to extensive habitat fragmentation (Choudhury, 2002). Notably, the Kokrajhar district has lost five fragmented populations over the past few decades (Biswas et al., 2019). Despite these setbacks, Kokrajhar district still retains six out of the twelve fragmented golden langur populations in India, harbouring roughly 15% of the national population (Biswas et al., 2024). These populations were historically part of a contiguous habitat network, but have since become isolated due to habitat fragmentation (Biswas et al., 2019), impeding population exchange and heightening the risk of local extinction (Frankham et al., 2004).
Despite their isolation, these populations still retain relatively high genetic diversity, likely due to recent isolation (Ram et al., 2016); however, they remain vulnerable to genetic fragmentation if the connectivity is not restored. Additionally, Aa substantial proportion of the golden langur population has begun adapting to human-altered environments, particularly village matrices (Medhi et al., 2004; Shil et al., 2021). The expansion of linear infrastructure within these fragmented habitats exacerbates these challenges by disrupting arboreal connectivity. This has resulted in increased road collisions, electrocutions, dog attacks, and mortality, and langurs straying into human settlements in search of food and refuge, all contributing to escalating human-langur conflict, further endangering the species (Chetry et al., 2020; Shil et al., 2021). These issues highlight the need for targeted conservation interventions to reduce risks and ensure the species’ survival (Shil et al., 2020). For several years, habitat fragmentation caused by linear infrastructure development has posed a significant threat to golden langurs and other arboreal primates elsewhere in Assam. In recent years, there has been a noticeable rise in incidents such as vehicle collisions, electrocutions, predator attacks, and langurs straying into human settlements in search of food and refuge, all contributing to escalating human-langur conflict (Biswas et al., 2019; Chetry et al., 2020; Shil et al., 2021). Thus, the need to facilitating their movements artificially across linear infrastructure like roads has become inevitable.
Different animals respond to such facilitation differently, e.g., elk took some time to use the newly created underpass in Arizona before using it regularly (Dodd et al., 2007).
We implemented different types of artificial canopy bridges and evaluated their effectiveness in mitigating the impact of linear infrastructure on golden langur in a fragmented habitat in Assam, India.
Materials and Methods
Study area
The study site encompasses the fragmented forest complex of the Kokrajhar district of Assam, India, viz. Nayekgaon Proposed Reserve Forest (PRF) & Rubber Garden, Buxamara Reserve Forest (RF), Amguri PRF, and Chakrashila Wildlife Sanctuary (WLS), which is situated in the southern periphery of its distribution range (Figure 2). Historically, these forest patches were part of a larger, contiguous forested landscape. However, over the years, they have become increasingly fragmented and isolated due to deforestation and land-use changes. A key linear infrastructure SH-14 of the Kokrajhar–Bahalpur road passes through the Nayekgaon PRF–Rubber Garden–Amguri–Buxamara stretch, effectively bisecting the Chakrashila WLS-Amguri–Buxamara forest from the Nayekgaon PRF-Rubber Garden and Nadangiri RF. The rubber garden, a privately owned plantation, served as an important corridor connecting Chakrashila WLS-Amguri–Buxamara with Nayekgaon and Nadangiri. The general forest types in the region are Assam Valley semi-evergreen forests, northern secondary moist mixed deciduous forests, moist plain Sal forests, and rubber gardens dominated by Sal (Shorea robusta) and rubber (Hevea brasiliensis) mixed with some semi-evergreen and evergreen species (Bahuguna et al., 2016).
Field methods:
Pre-ACB installation: We stationed three observers, comprising one research assistant and two community volunteers, on the road (SH-14) to monitor the golden langur movement along the road from January 2023 to November 2023. The observers recorded data between 07:00 hours and 17:00 hours, 16 to 20 days in a month, amounting to a total of 1768 hours of survey effort in this period. Once the langur group was spotted along the road, the group was observed until they moved away from the road. The time of crossing, number of individuals, path taken, substrate used, and the height of the animal during their movement were recorded.
Observers were stationed from June to August, 2023, two days a month, to signal passing the vehicles passing at high speed to reduce their speed at an accident-prone area, where there were high possibilities of animal crossings. Observers signalled a total of 50 vehicles when golden langur groups were active close to the roadside or attempting to cross and recorded the responses of the commuters. Vehicles’ speed compliances were observed visually without a speedometer. Speed compliances were considered positive when vehicles used a full break or decreased their speed significantly. In September 2023, we erected two signage at both ends of the accident-prone area of the road to educate and signal the traffic. We further continued signalling vehicles to reduce their speed for two days a month, from September to November 2023. For comparison, observers signalled to another 50 vehicles while golden langur groups were near the roadside, keeping the signage in focus.
We recorded every langur collision with vehicles and other causes of their death along the road. Using this information, we identified the crucial location of animal crossings and possible animal collision sites. (Figure 6a and Figure 6b).
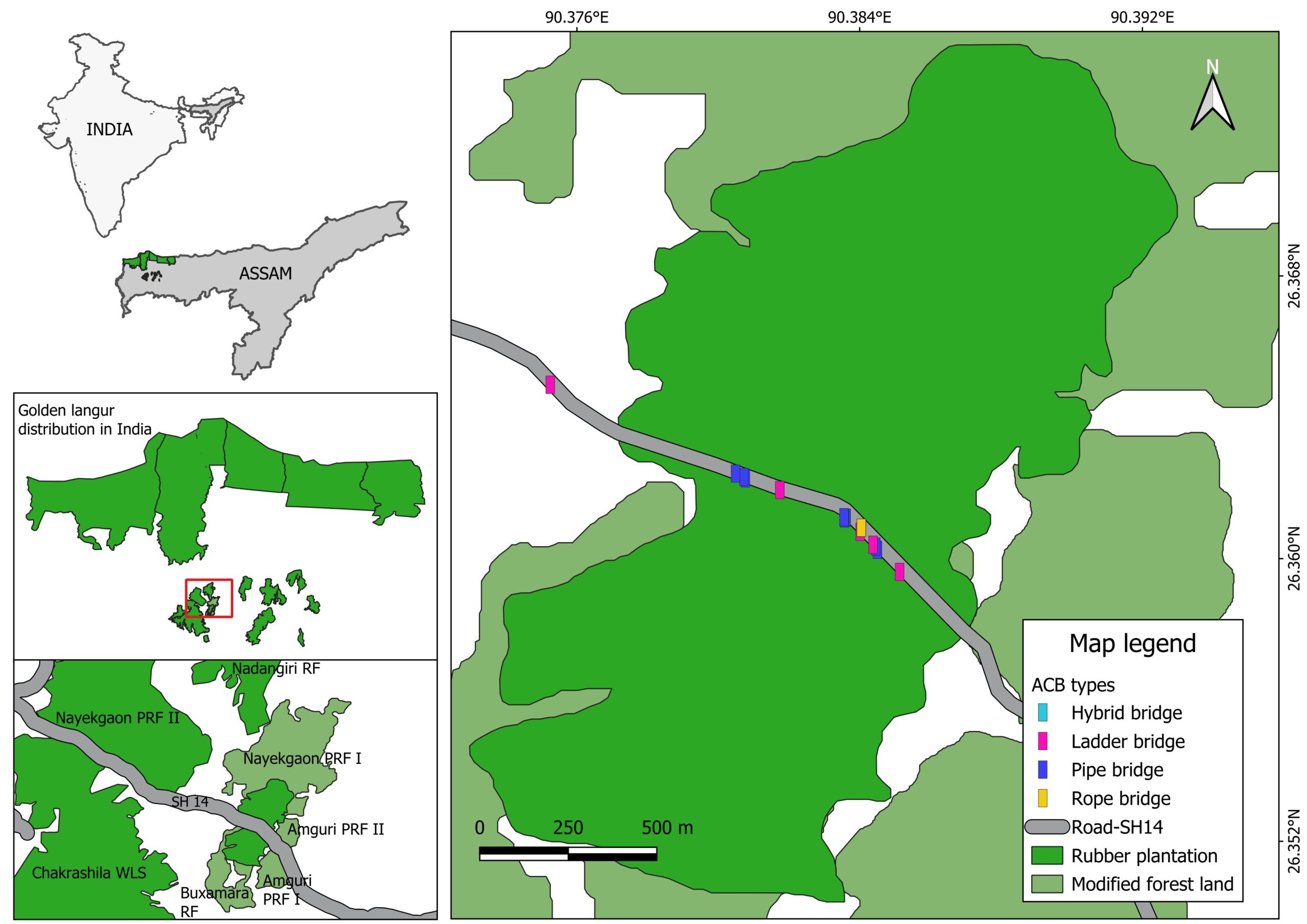
Figure 2: Fragmented forest habitats of the golden langur in Kokrajhar district, Assam, India, showing the locations of artificial canopy bridges (ACB).
Installation of ACB: To construct the ACBs, we assessed the parameters like bridge length, anchor trees, canopy height, angle of the bridges, owner of the area, bridge type, and bridge viability to select the appropriate sites in the selected crucial locations. Considering the durability, strength, flexibility, and suitability for arboreal primates, we selected, i) 2.54 cm thick unplasticized polyvinyl chloride (UPVC) pipes, caps, ii) tie cables of varying size and strength, iii) insulated gym cables of varying size (6 mm, 5 mm & 4 mm), iv) adhesive, both side glue tape, v) 5.08 cm diameter high density polyethylene (HDPE) pipes, vi) 6 mm galvanizsed cable and 0.75mm steel wire, vii) 2.4 cm diameter nylon ropes, viii) bamboo poles and bamboo tubes, and ix) 20 mm insulated aluminium cable.
Bridge selection and construction: We initially used bamboo bridges to acclimatise the langurs to use the canopy bridges and later replaced this with the pipe bridges and ladder bridges (Table 1).
I. Bamboo bridge:
a. A 15-meter-long mature bamboo pole was installed on both anchor trees above the power line and securely fastened between them.
b. Bamboo poles measuring 3 cm in diameter were sliced into 10 cm lengths. These pieces were then threaded onto a 2.4 mm diameter nylon rope, forming a flexible yet sturdy bamboo chain. Once assembled, the rope cum bamboo chain bridge was installed between two anchor trees by securely fastening it to the branches. (Figure 6c)
II. Rope bridge: The bridges were constructed using thick yellow coloured nylon rope with a diameter of 2.4 cm, securely tied to pre-identified anchor trees. The ropes were fastened to branches at a height of approximately 1.5 to 2 meters above the electric lines crossing the road, thereby eliminating any risk of electrocution. (Figure 6d)
III. Pipe bridge: The bridges were constructed with HDPE pipes (5.08 cm diameter, typically used for water supply), combined with 2.4 cm diameter nylon rope and 4 mm galvanised wire. Considering the width of the canopy gap, the HDPE pipe was cut to the required length. A 2.4 cm diameter nylon rope and a 4 mm galvanised wire were inserted through the pipe, with an excess length of 6–8 meters on either end to allow the pipe to secure on the anchor trees. Two small holes were drilled at both ends and every 4 m of the HDPE pipe. The inserted rope and galvanised wire were tightly secured to the pipe with 0.75 mm steel wire at the drilled holes of the pipe. This provided additional stability and ensured that the rope remained firmly in place within the pipe. The bridge was lifted and positioned across the preselected canopy gap. The ropes were then securely fastened to strong branches on each side of the designated anchor trees. A total of eight pipe bridges were installed at the identified locations. (Figure 6e)
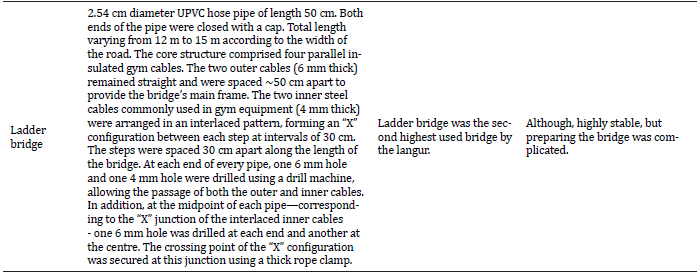
IV. Ladder bridge: We used a 2.54 cm diameter UPVC hose pipe of 50 cm length. Both ends of the pipe were closed with a cap using UPVC joining solvent. The bridge was constructed in the style of a horizontal “ship” ladder, with its total length varying from 12 m to 15 m according to the width of the road. The core structure comprised four parallel insulated gym cables. The two outer cables (6 mm thick) remained straight and were spaced approximately 50 cm apart to provide the bridge’s main frame. The two inner steel cables (4 mm thick), that are commonly used in gym equipment, were arranged in an interlaced pattern, forming an “X” configuration between each step, spaced at intervals of 30 cm along the length of the bridge (see Figure 3a). At each end of every pipe, one 6 mm hole and one 4 mm hole were drilled using a drill machine, allowing the passage of both the outer and inner cables. In addition, at the midpoint of each pipe—corresponding to the “X” junction of the interlaced inner cables – one 6 mm hole was drilled at each end and another at the centre. The crossing point of the “X” configuration was secured at this junction using a thick rope clamp, ensuring overall stability and preventing cable movement during use. We constructed five ladder bridges in Siljan, Kokrajhar. (Figure 6f).
All of the bridges were placed at a height of nine to ten meters from the ground (Figure 3b).
Monitoring of ACB: Following the installation of all bridges, three observers and two community volunteers monitored the bridges from mid -December 2023 to December 2024, for the maintenance and management of the canopy bridges. They monitored the road crossings by langurs using ACBs and other crossing locations. Observers also periodically walked on either side of the road from the ACB for about 1.5 km to monitor the groups. The observers recorded data between 07:00 hours and 17:00 hours, ~ 20 days in a month, resulting in a total survey effort of 2216 hours. When a group of golden langur was detected crossing the road, the observers recorded date, start and end timings of the observation, group size, location, and crossing pattern.
We deployed 13 camera traps, comprising two Spartan cameras, five Reconyx cameras, and six Cuddeback cameras, across all ACB sites alternatively. All the camera traps were active simultaneously during the survey period without any gap.
We compared the number of langur deaths due to electrocutions, and collisions with vehicles before (January 2023 to November 2023) and after installing the canopy bridges (mid-December 2023 to December 2024). We compared the responses of vehicles to signalling and signages asking them to slow down to avoid a collision. We compared the number of road crossings made by the langurs using different canopy bridges using the Chi-squared test. We used QGIS 3.42 (QGIS Development Team, 2025) for map creation and used Python 3.13 (Python Software Foundation, 2025) for statistical analysis.
Results
When the langurs were on the roadside, observers signalled the high-speed vehicles (n = 50) to slow down. The signals were disregarded 92% (n = 46) of the time. The persisted signalling with signage resulted in an increased response from 8% to 18% by the moving vehicles. We identified 18 critical crossover points by the golden langurs on SH-14 on a 5.2 km stretch from Nayekgaon to Choibari. These crossover points were used by eight groups of golden langurs, of which seven were mixed groups while one was an all-male band. Before installing the canopy bridges, golden langurs crossed the road by walking on the ground on 71% of the occasions, while they altered their route to use the existing natural canopy on 29% of the occasions.
We installed 15 canopy bridges along the SH-14, comprising eight pipe bridges, five ladder bridges, one rope bridge and one hybrid bridge (developed by replacing the bamboo-pole bridge) (Table 1). The time taken by the langurs to get habituated to using the different ACBs varied depending on the materials used; however, they eventually adapted to using them. Golden langurs habituated to the bamboo pole bridge and rope bridge within 15 and 23 days of installation (Figure 6g & 8h), respectively. After the acclimatisation phase, the bamboo-pole bridge was replaced with a mixed bamboo-and-rope structure (hybrid bridge). In contrast, pipe bridges took slightly longer, with an average habituation period of 29 days (Figure 6i). During the acclimatisation phase, golden langurs quickly started using the bamboo canopy bridges over the rope bridge (Figure 6h). Ladder bridges initially required a longer habituation period
Table 1. Description of different canopy bridges, their limitations and advantages and the response of langurs.

(a) 
(b) 
Figure 3: (a) Top view of the ladder bridge with the measurements. (b) Design for the ladder bridge for golden langurs.
nearly 90 days before the first observed crossing but this duration decreased to ~ 65 days in subsequent installations (Figure 6j).
We recorded 112 instances of golden langurs crossing the road during the monitoring period. Langurs used the canopy bridges (74.0 %, n = 83) significantly more than the road (25.8 %, n = 29) (χ2 = 26.04, df = 1, p < 0.01). Further, out of 83 crossings using canopy bridges, 69.9 % (n = 58) were using pipe bridges, 26.5 % (n = 22) of the crossings were using ladder bridges, 2.4% (n = 2) of the crossings were using hybrid bridges and 1.2 % (n = 1) of the crossings were using rope bridges, (χ2 = 102.69, df = 3, p < 0.001) (Figure 4).
Between January 2023 and December 2024, we documented a total of 18 golden langur road collisions on SH-14 (Figure 5a). Of those, 11 collisions leading to five deaths occurred before installing the canopy bridges, while two deaths due to seven collisions occurred after installing the canopy bridges. Out of 18 collision incidents in total, langurs were killed in seven incidents, sustained major injuries, that mainly included broken legs or the amputation of palms or tails in five incidents, and langurs escaped with minor injuries in the other six incidents. Meanwhile, there were three deaths of langurs in the five electrocution incidents in the period of pre canopy bridge installations, whereas we recorded five deaths in seven electrocution incidents in the period of post canopy bridge installations where electric power lines were not insulated.
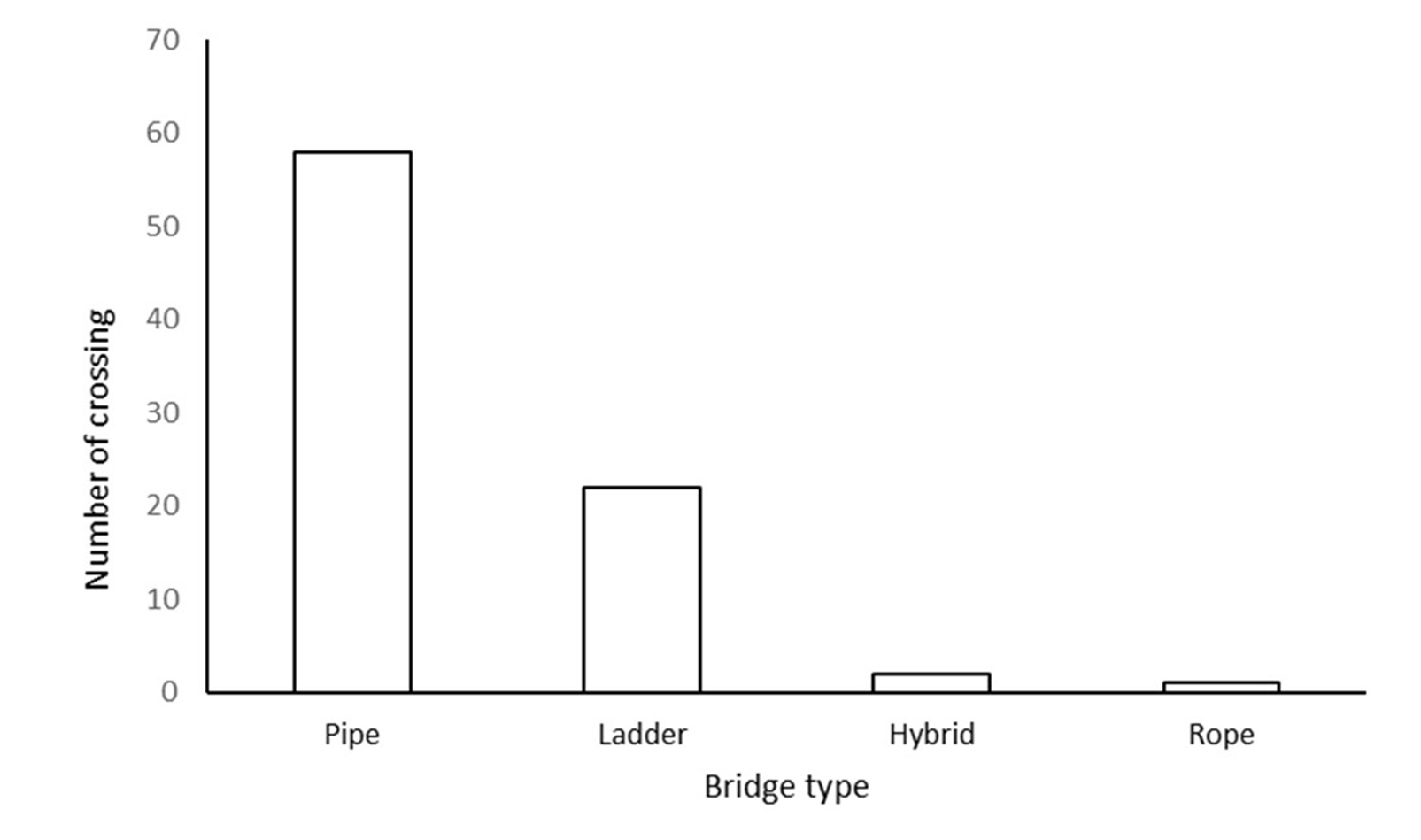
Figure 4: Frequency of use for different designs of artificial canopy bridges by the golden langur in Kokrajhar district, Assam, India.
Discussion
Artificial canopy bridges (ACBs) substantially improved golden langur crossing by reducing their collisions with vehicles on 5.2 km stretch of SH-14 between Nayekgaon to Choibari. ACBs are increasingly used as a practical mitigation tool to restore connectivity, reduce mortality, and support conservation. Their effectiveness, however, depends on species-specific design, proper placement, and rigorous evaluation (Soanes et al., 2024; van der Grift & van der Ree, 2015). In Australia, squirrel gliders (Petaurus norfolcensis) initially re-established movement using rope bridges and glide-poles (Soanes et al., 2013). Later genetic analyses showed restored gene flow within five years, proving that aerial structures can reverse population fragmentation (Soanes et al., 2018). The critically endangered Hainan gibbon (Nomascus hainanus) rapidly adapted to a rope bridge across a landslide, moving naturally and safely in Hainan, China (Chan et al., 2020). In Kenya, “Colobridges” reduced road mortality among primates and proved sustainable with community involvement (Cunneyworth et al., 2022). In Bangladesh, rare use of canopy bridges by slow lorises (Nycticebus bengalensis) confirmed that even cryptic, nocturnal species can benefit from ACBs (Maria et al., 2022). After recording the high mortality of lion-tailed macaque (Macaca silenus) in
(a)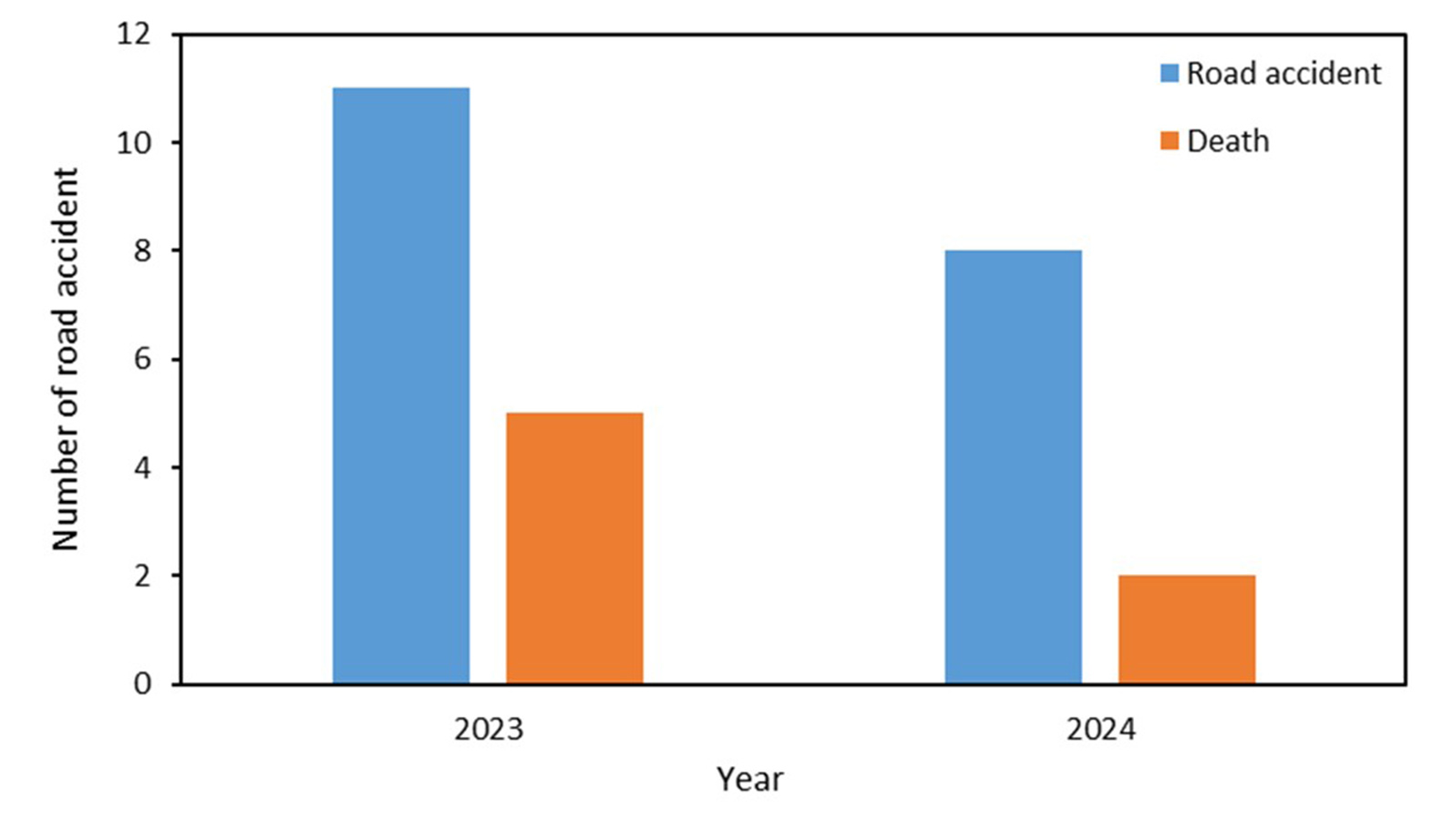
(b) 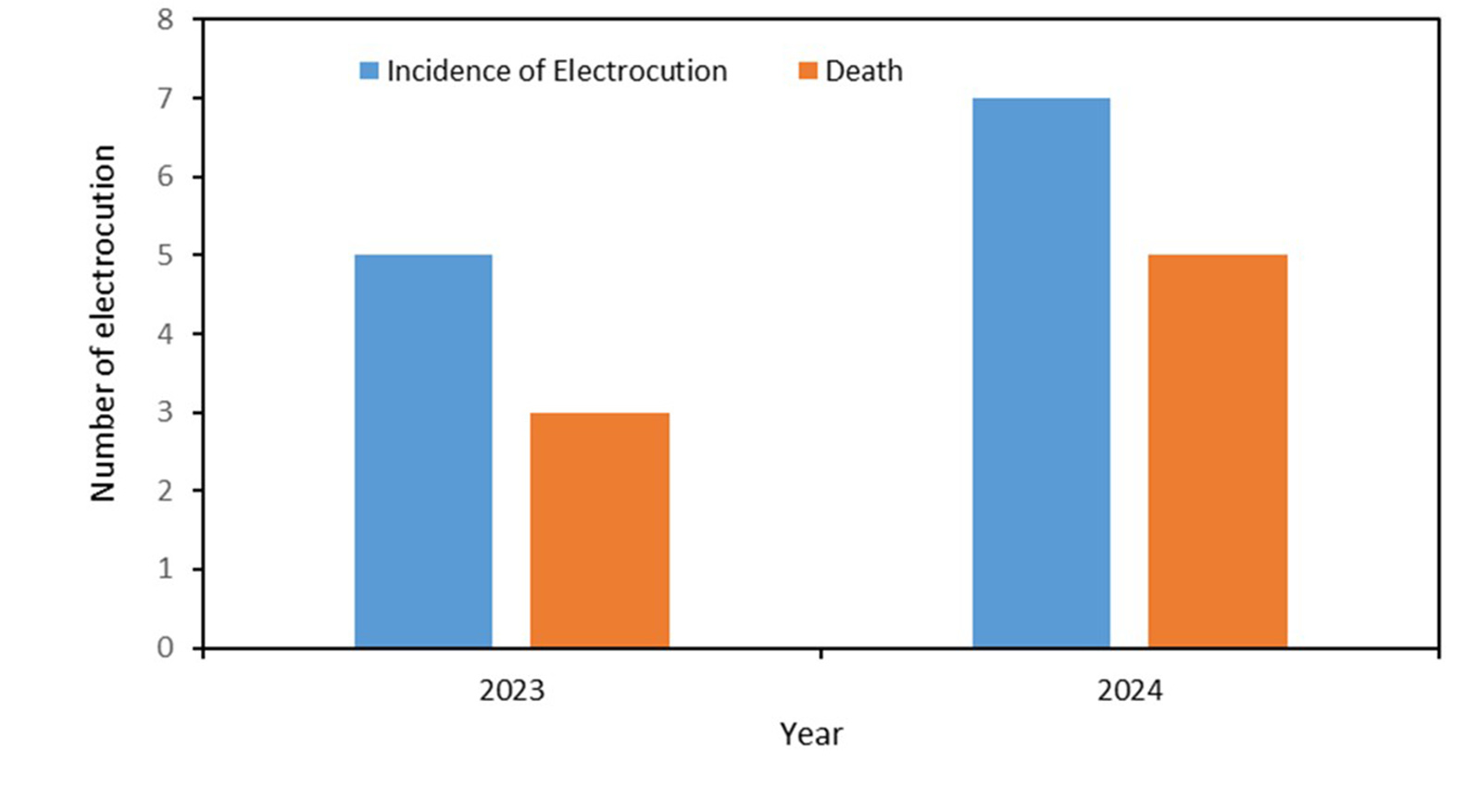
Figure 5: (a) Number of langur collisions with vehicles and deaths that occurred during the period of pre and post canopy bridge installations in Kokrajhar district, Assam, India. (b) Number of langur electrocution and their deaths during the period of pre and post canopy bridge installations in Kokrajhar district, Assam, India.
Puthuthotam in Anamalai Hills, a few canopy bridges were installed (Jeganathan et al., 2018), however, the estimation of their efficiency in reducing the ground movement of lion-tailed macaque and change in the rate of their roadkill is not available. At Hollongapar Gibbon Wildlife Sanctuary (Assam), targeted planting created a natural canopy bridge, later used by western hoolock gibbons (Hoolock hoolock) (Chetry et al., 2022). Earlier work in Assam used ACBs for gibbons as temporary measures until forest regeneration closed canopy gaps (Das et al., 2009). These evidence further reinforce the importance of ACBs in crucial crossovers of the animals.
ACBs will be efficient when integrated into wider landscape strategies: vegetation restoration to replace and complement artificial with natural bridges, speed management at hotspots, and power line insulation to reduce electrocutions (Chetry et al., 2022; Clevenger & Huijser, 2011). Evidence suggests canopy bridges are relatively low-cost, community-manageable, and effective when combined with habitat restoration (Cunneyworth et al., 2022). We observed that when only a nominal number of bridges were available, golden langurs tended to avoid using them, likely due to limited options. However, as the number and spatial distribution of artificial structures increased, providing a wider range of crossing points, the frequency of usage substantially increased. Ladder bridges initially required a longer habituation period, but this duration decreased in subsequent installations, suggesting that prior exposure helped facilitate faster adaptation.
There are some challenges we encountered in the implementation of the ACBs. During the study, five artificial canopy bridges were planned along SH-14 for golden langur crossings. However, the clearance of a rubber garden, which served as a habitat and transit route, led to the displacement of langur groups. This increased their ground crossings, collision risks, and straying into villages, highlighting the need for additional ACBs to mitigate these threats.

Figure 6 (A-J)
We facilitated the insulation of power lines in critical crossover stretches through the electricity department, which not only minimised electrocution risks but also facilitated their movement. However, the langurs have since become habituated to using power lines more confidently and now frequently move beyond the insulated stretches, where bare power lines pass through, leading to increased incidents of electrocution-related deaths and injuries (Figure 5b). For species such as the golden langur, whose substantial population (25%) is confined to fragmented habitats (Biswas et al., 2024), ACBs provide immediate risk reduction while long-term restoration proceeds. For this, we have engaged local communities in joint monitoring of ACBs and creating natural canopies through extensive plantation of native tree species along critical stretches of the road and by restoring green corridors in the backyards. The initiative integrates education and community outreach to promote sustainable livelihoods, offering incentives for corridor maintenance and conflict mitigation. Multi-year monitoring and genetic analysis, where feasible, should be standard, ensuring that ACBs contribute not only to safe crossings but also to long-term conservation and connectivity (Soanes et al., 2018; Soanes et al., 2024).
Acknowledgement
We are thankful to the Department of Environment and Forest, Government of Assam, particularly the then PCCF (Wildlife) and Chief Wildlife Warden, Assam, Mr. Sandeep Kumar, the Council Head of the Department of Forest, BTC, Mr. Suman Mohapatra and Mr. Jayanta Kr Brahma, Divisional Forest Officer – Haltugaon Forest Division for providing the necessary permissions and logistical support. We gratefully acknowledge Dr. GV Gopi, Scientist-F and Head, Department of Endangered Species Management, Wildlife Institute of India for approving our designs. We also acknowledge the Range Forest Officer and forest personnel of Nayekgaon Range of Haltugaon Forest Division and Kokrajhar Wildlife Division for their assistance during fieldwork. We extend our gratitude to Ms. Bijoya Boro, General Manager, and all staff of the Assam Power Distribution Corporation Limited, as well as Mr. Simanta Das, Manager and all other staffs of Abhaya Rubber Plantation, for their generous support. We also thank the local communities residing in the fringe areas of the forest complex for their cooperation throughout the fieldwork. We are also deeply indebted to Dr. Santosh Kumar Sahoo and Ms. Aruna Negi of Conservation Himalayas for their support. Special thanks go to our dedicated field workers, particularly Mr. Bishop Kujur, Mr. Kamaleswar Sangma, Mr. Tipendra Sangma and Mr. Chandan Mahato, whose efforts were invaluable during construction and installation phase of the artificial canopy bridges.
This study was initiated with a small support from the BTC Forest Department, Kokrajhar. We extend our sincere gratitude to PTES for their partnership and generous financial support, which was instrumental in the successful implementation of this program. We also gratefully acknowledge additional support from BAT under their Coexistence SGP program, as well as from Re-Wild, CBOT, and MBZSCF. All research protocols reported in this manuscript were reviewed and approved by the Primate Research Centre NE India, Conservation Himalayas, and the Salim Ali Centre for Ornithology and Natural History.
ETHICS STATEMENT
The study was non-invasive and followed the guidelines for Best Practices for field Primatology. The research complied with a protocol approved by the Principal Chief Conservator of Forests and Chief Wildlife Warden, Assam, Council Head of the Department, Bodoland Territorial Council, and approved by the internal research monitoring committee, SACON (WII). The research adhered to the legal requirements of the Forest Department, Assam. The authors have no conflict of interest to declare.
CONFLICT OF INTEREST
Dr. H.N. Kumara holds editorial positions at the Journal of Wildlife Science. However, he did not participate in the peer review process of this article except as an author. The authors declare no other conflict of interest.
DATA AVAILABILITY
Data and codes are available from the corresponding author on request. All designs and data are the sole property of PRCNE.
AUTHORS’ CONTRIBUTION
Jihosuo Biswas: Conceptualization, Fund and resource raising, Designing, Project ad-ministration, Data curation, Implementation, Supervision, Investigation, Formal analy¬sis, Writing – original draft, review & editing.
Joydeep Shil: Data curation, Implementation, Supervision, Investigation, Formal analy¬sis, Writing – original draft, review & editing.
Kanmaina Ray: Data curation, Investigation, Implementation.
Mehtab Uddin Ahmed: Supervision, Data curation, Implementation.
Dharma Kanta Ray: Data curation, Implementation.
Amulya Boro: Data curation, Implementation.
Puja Muchahary: Data curation, Implementation.
Benjamin P Dorsey: Implementation, review & editing.
Honnavalli Kumara: Formal analysis, Writing – review & editing, Validation.
USE OF GENERATIVE AI STATEMENT
We declare that we have not used AI software for writing or analysing data for this article.
ORIGINALITY STATEMENT
We declare that this manuscript represents original research, without duplication from other studies, the published work of the authors or that it is not a salami-slicing paper from other major work by the author.
Edited By
Mewa Singh
University of Mysore, Mysore, India.
*CORRESPONDENCE
Jihosuo Biswas
✉ jihosuo@yahoo.com
Joydeep Shil
✉ joydshil@gmail.com
CITATION
Biswas, J., Shil, J., Ray, K., Ahmed, M. U., Ray, D. K., Boro, A., Muchahary, P., Dorsey, B. & Kumara, H. N. (2025). Mitigating linear infrastructure: Artificial canopy bridges as a key mitigating tool to reduce golden langur (Trachypithecus geei) road accidents in Assam, India. Journal of Wildlife Science, 2(4), 104-113. https://doi.org/10.63033/JWLS.SZBG7452
FUNDING
This work was supported in part by the Bodoland Territorial Council Forest Department of Assam, People’s Trust for Endangered Species, British Asia Trust, Re-Wild, CBOT and MBZSCF. The funding agencies were not involved in the design of the study, data collection, data analysis, decision to publish or preparation of any part of the manuscript.
COPYRIGHT
© 2025 Biswas, Shil, Ray, Ahmed, Ray, Boro, Muchahary, Dorsey & Kumara. This is an open-access article, immediately and freely available to read, download, and share. The information contained in this article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), allowing for unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited in accordance with accepted academic practice. Copyright is retained by the author(s).
PUBLISHED BY
Wildlife Institute of India, Dehradun, 248 001 INDIA
PUBLISHER'S NOTE
The Publisher, Journal of Wildlife Science or Editors cannot be held responsible for any errors or consequences arising from the use of the information contained in this article. All claims expressed in this article are solely those of the author(s) and do not necessarily represent those of their affiliated organisations or those of the publisher, the editors and the reviewers. Any product that may be evaluated or used in this article or claim made by its manufacturer is not guaranteed or endorsed by the publisher.
Asensio, N., Kachanan, J., Saralamba, C. & José-Domínguez, J. M. (2021). The impact of roads on the movement of arboreal fauna in protected areas: the case of lar and pileated gibbons in Khao Yai National Park, Thailand. Journal of Tropical Ecology, 37(6), 276-285. https://doi.org/10.1017/S0266467421000390
Bahuguna, V. K., Swaminath, M. H., Tripathi, S., Singh, T. P., Rawat, V. R. S., & Rawat, R. S. (2016). Revisiting forest types of India. International Forestry Review, 18(2), 135-145.
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? in L.K. Marsh (ed.), Primates in Fragments: Ecology and Conservation, Kluwer Academics/Plenum Press, New York, USA, 283–303. https://doi.org/10.1007/978-1-4757-3770-7_18
Biswas, J. (2002). Ecology and social behaviour of golden langur (Trachypithecus geei) Khajuria, 1956. PhD thesis, Gauhati University, Guwahati, p.452.
Biswas, J., Shil, J., Nag, S. & Bhattacharjee, P. C. (2019). Consequences of habitat fragmentation on the Socio-Ecology and gastrointestinal parasitic load of Golden langur in Assam, India. Technical Report PRC/32, Primate Research Centre, 109 Nehru Park, Jodhpur, Rajasthan, India. Submitted to SERB-DST, Government of India, p.102.
Biswas, J., Shil, J., Sasi, R., Ahmed, M. U., Barman, K., Das, N., Basumatary, B. & Kumara, H. N. (2024). Ecological determinants of occupancy of golden langur Trachypithecus geei and its population characteristics in India, Global Ecology and Conservation, 53, e03003. https://doi.org/10.1016/j.gecco.2024.e03003
Chan, B. P. L., Tan, C. K. W., Hamblin-Jones, B. & Fellowes, J. R. (2020). First use of artificial canopy bridge by the world's most critically endangered primate: Hainan gibbon (Nomascus hainanus), Scientific Reports, 10, 15284. https://doi.org/10.1038/s41598-020-72641-z
Chapman, C. A., Wasserman, M. D., Gillespie, T. R., Speirs, M. L., Lawes, M. J., Saj, T. L. & Ziegler, T. E. (2006). Do food availability, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? American Journal of Physical Anthropology, 131(4), 525–534. https://doi.org/10.1002/ajpa.20477
Chetry, D., Phukan, M., Chetry, R., Boro, R.N., Das, A. K. & Bhattacharjee, P. C. (2020). Conservation Status of the Golden Langur Trachypithecus geei in Chakrashila Wildlife Sanctuary, Assam, India, Primate Conservation, 34, pp.167–173.
Chetry, R., Chetry, D., Singh, M., Das, N. & Medhi, R. (2022). A natural canopy bridge at the Hollongapar Gibbon Wildlife Sanctuary, Assam, India, Primate Conservation, 36, pp.47–55.
Choudhury, A. (2002). Golden langur Trachypithecus geei threatened by habitat fragmentation. ZOOS' Print Journal, 17(2), 699-703. https://doi.org/10.11609/JoTT.ZPJ.17.2.699-703
Clevenger, A. P. & Huijser, M. P. (2011). Wildlife Crossing Structure Handbook: Design and Evaluation in North America. Publication No. FHWA-CFL/TD-11-003, U.S. Federal Highway Administration, Washington, DC.
Cunneyworth, P. M., Donaldson, A., & Onyancha, F. (2022). Canopy bridges are an economical mitigation reducing the road barrier effect for three of four species of monkeys in Diani, Kenya. Folia Primatologica, 93(3-6), 217-234.
Das, J., Biswas, J., Bhattacherjee, P. C. & Rao, S. S. (2009). Canopy Bridges: An Effective Conservation Tactic for Supporting Gibbon Populations in Forest Fragments, in S. Lappan & D. J. Whittaker (eds.), The Gibbons. Developments in Primatology: Progress and Prospects, Springer, New York, NY, pp.467–475. https://doi.org/10.1007/978-0-387-88604-6_22
Dodd, N. L., Gagnon, J. W., Boe, S., Manzo, A. & Schweinsburg, R. E. (2007). Evaluation of measures to minimize wildlife-vehicle collisions and maintain permeability across highways: Arizona Route 260. Final Report, 540. Arizona Game and Fish Department, Research Branch, Arizona, USA.
Donaldson, A. & Cunneyworth, P. (2015). Case study: canopy bridges for primate conservation, in R. van der Ree, D. J. Smith & C. Grilo (eds.), Handbook of Road Ecology, John Wiley & Sons, West Sussex, UK, pp.341–343. https://doi.org/10.1002/9781118568170.ch41
Frankham, R., Ballou, J. D. & Briscoe, D. A. (2004). A Primer of Conservation Genetics, Cambridge University Press, p.236. https://doi.org/10.1017/CBO9780511817359
Jeganathan, P., Mudappa, D., Raman, T. S. & Kumar, M. A. (2018). Understanding perceptions of people towards lion-tailed macaques in a fragmented landscape of the Anamalai Hills, Western Ghats, India. Primate Conservation, 32(11), 205-215.
Lokschin, L. X., Printes, R. C., Cabral, J. N. H. & Buss, G. (2007). Power lines and howler monkey conservation in Porto Alegre, Rio Grande do Sul, Brazil, Neotropical Primates, 14(2), 76–80. https://doi.org/10.1896/044.014.0206
Maria, M., Al-Razi, H., Ali, A., Muzaffar, S. B. & Nekaris, K. A. I. (2022). Artificialcanopy bridge use by primates and other arboreal mammals in a fragmented tropical forest of northeast Bangladesh, Folia Primatologica, 93(3-6), 217–234. https://doi.org/10.1163/14219980-20211201
Mass, V., Rakotomanga, B., Rakotondratsimba, G., Razafindramisa, S., Andrianaivomahefa, P., Dickinson, S. & Cooke, A. (2011). Lemur bridges provide crossing structures over roads within a forested mining concession near Moramanga, Toamasina Province, Madagascar, Conservation Evidence, 8, 11–18.
Medhi, R., Chetry, D., Bhattacharjee, P. C. & Patiri, B. N. (2004). Status of Trachypithecus geei in a rubber plantation in Western Assam, India, International Journal of Primatology, 25(6), 1331–1337. https://doi.org/10.1023/B:IJOP.0000043965.38722.63
Onderdonk, D. A. & Chapman, C. A. (2000). Coping with forest fragmentation: the primates of Kibale National Park, Uganda, International Journal of Primatology, 21(4), 587–611. https://doi.org/10.1023/A:1005509119693
Praill, L. C., Eppley, T. M., Shanee, S., Cunneyworth, P. M. K., Abra, F. D., Allgas, N., Al-Razi, H., Campera, M., Cheyne, S. M. et al. (2023). Road Infrastructure and Primate Conservation: Introducing the Global Primate Roadkill Database, Animals, 13(10), 1692. https://doi.org/10.3390/ani13101692
Python Software Foundation (2025). Python (Version 3.13). Available at: https://www.python.org
QGIS Development Team (2025). QGIS Geographic Information System (Version 3.42). https://qgis.org
Ram, M. S., Kittur, S. M., Biswas, J., Nag, S., Shil, J. & Umapathy, G. (2016). Genetic diversity and structure among isolated populations of the endangered Gee’s golden langur in Assam, India, PLoS One, 11(8), e0161866. https://doi.org/10.1371/journal.pone.0161866
Shil, J., Biswas, J. & Kumara, H. N. (2020). Influence of habitat conditions on group size, social organization, and birth pattern of golden langur (Trachypithecus geei), Primates, 61(6), 797–806. https://doi.org/10.1007/s10329-020-00829-y
Shil, J., Biswas, J., Nag, S. & Kumara, H. N. (2021). Persistence of Trachypithecus geei (Mammalia: Primates: Cercopithecidae) in a rubber garden in Assam, India, Journal of Threatened Taxa, 13(7), 18679–18686. https://doi.org/10.11609/jott.7273.13.7.18679-18686
Soanes, K., Goldingay, R. & van der Ree, R. (2013). Movement re-established but not restored: inferring the effectiveness of built crossing structures for gliding mammals, Biological Conservation, 159, 86–93. https://doi.org/10.1016/j.biocon.2012.10.016
Soanes, K., Rytwinski, T., Fahrig, L., Huijser, M. P., Jaeger, J. A. G., Teixeira, F. Z., van der Ree, R. & van der Grift, E. A. (2024). Do wildlife crossing structures mitigate the barrier effect of roads on animal movement? A global assessment, Journal of Applied Ecology, 61(2), 271–289. https://doi.org/10.1111/1365-2664.14582
Soanes, K., Taylor, A. C., Sunnucks, P., Vesk, P. A., Cesarini, S. & van der Ree, R. (2018). Evaluating the success of wildlife crossing structures using genetic approaches and an experimental design: lessons from a gliding mammal, Journal of Applied Ecology, 55(1), 129–138. https://doi.org/10.1111/1365-2664.12966
Srivastava, A., Biswas, J., Das, J. & Bujarbarua, P. (2001). Status and distribution of golden langurs (Trachypithecus geei) in Assam, India, American Journal of Primatology, 55(1), 15–23. https://doi.org/10.1002/ajp.1035
Thinley, P., Norbu, T., Rajaratnam, R., Vernes, K., Wangchuk, K., Choki, K., Tenzin, J., Tenzin, S. & Kinley et al. (2019). Population abundance and distribution of the endangered golden langur (Trachypithecus geei, Khajuria 1956) in Bhutan, Primates, 60(5), 437-448. https://doi.org/10.1007/s10329-019-00737-w
van der Grift, E. A. & van der Ree, R. (2015). Guidelines for evaluating use of wildlife crossing structures, in R. van der Ree, D. J. Smith & C. Grilo (eds.), Handbook of Road Ecology, John Wiley & Sons, Chichester, 411–419. https://doi.org/10.1002/9781118568170.ch15
Edited By
Mewa Singh
University of Mysore, Mysore, India.
*CORRESPONDENCE
Jihosuo Biswas
✉ jihosuo@yahoo.com
Joydeep Shil
✉ joydshil@gmail.com
CITATION
Biswas, J., Shil, J., Ray, K., Ahmed, M. U., Ray, D. K., Boro, A., Muchahary, P., Dorsey, B. & Kumara, H. N. (2025). Mitigating linear infrastructure: Artificial canopy bridges as a key mitigating tool to reduce golden langur (Trachypithecus geei) road accidents in Assam, India. Journal of Wildlife Science, 2(4), 104-113. https://doi.org/10.63033/JWLS.SZBG7452
FUNDING
This work was supported in part by the Bodoland Territorial Council Forest Department of Assam, People’s Trust for Endangered Species, British Asia Trust, Re-Wild, CBOT and MBZSCF. The funding agencies were not involved in the design of the study, data collection, data analysis, decision to publish or preparation of any part of the manuscript.
COPYRIGHT
© 2025 Biswas, Shil, Ray, Ahmed, Ray, Boro, Muchahary, Dorsey & Kumara. This is an open-access article, immediately and freely available to read, download, and share. The information contained in this article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), allowing for unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited in accordance with accepted academic practice. Copyright is retained by the author(s).
PUBLISHED BY
Wildlife Institute of India, Dehradun, 248 001 INDIA
PUBLISHER'S NOTE
The Publisher, Journal of Wildlife Science or Editors cannot be held responsible for any errors or consequences arising from the use of the information contained in this article. All claims expressed in this article are solely those of the author(s) and do not necessarily represent those of their affiliated organisations or those of the publisher, the editors and the reviewers. Any product that may be evaluated or used in this article or claim made by its manufacturer is not guaranteed or endorsed by the publisher.
Asensio, N., Kachanan, J., Saralamba, C. & José-Domínguez, J. M. (2021). The impact of roads on the movement of arboreal fauna in protected areas: the case of lar and pileated gibbons in Khao Yai National Park, Thailand. Journal of Tropical Ecology, 37(6), 276-285. https://doi.org/10.1017/S0266467421000390
Bahuguna, V. K., Swaminath, M. H., Tripathi, S., Singh, T. P., Rawat, V. R. S., & Rawat, R. S. (2016). Revisiting forest types of India. International Forestry Review, 18(2), 135-145.
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? in L.K. Marsh (ed.), Primates in Fragments: Ecology and Conservation, Kluwer Academics/Plenum Press, New York, USA, 283–303. https://doi.org/10.1007/978-1-4757-3770-7_18
Biswas, J. (2002). Ecology and social behaviour of golden langur (Trachypithecus geei) Khajuria, 1956. PhD thesis, Gauhati University, Guwahati, p.452.
Biswas, J., Shil, J., Nag, S. & Bhattacharjee, P. C. (2019). Consequences of habitat fragmentation on the Socio-Ecology and gastrointestinal parasitic load of Golden langur in Assam, India. Technical Report PRC/32, Primate Research Centre, 109 Nehru Park, Jodhpur, Rajasthan, India. Submitted to SERB-DST, Government of India, p.102.
Biswas, J., Shil, J., Sasi, R., Ahmed, M. U., Barman, K., Das, N., Basumatary, B. & Kumara, H. N. (2024). Ecological determinants of occupancy of golden langur Trachypithecus geei and its population characteristics in India, Global Ecology and Conservation, 53, e03003. https://doi.org/10.1016/j.gecco.2024.e03003
Chan, B. P. L., Tan, C. K. W., Hamblin-Jones, B. & Fellowes, J. R. (2020). First use of artificial canopy bridge by the world's most critically endangered primate: Hainan gibbon (Nomascus hainanus), Scientific Reports, 10, 15284. https://doi.org/10.1038/s41598-020-72641-z
Chapman, C. A., Wasserman, M. D., Gillespie, T. R., Speirs, M. L., Lawes, M. J., Saj, T. L. & Ziegler, T. E. (2006). Do food availability, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? American Journal of Physical Anthropology, 131(4), 525–534. https://doi.org/10.1002/ajpa.20477
Chetry, D., Phukan, M., Chetry, R., Boro, R.N., Das, A. K. & Bhattacharjee, P. C. (2020). Conservation Status of the Golden Langur Trachypithecus geei in Chakrashila Wildlife Sanctuary, Assam, India, Primate Conservation, 34, pp.167–173.
Chetry, R., Chetry, D., Singh, M., Das, N. & Medhi, R. (2022). A natural canopy bridge at the Hollongapar Gibbon Wildlife Sanctuary, Assam, India, Primate Conservation, 36, pp.47–55.
Choudhury, A. (2002). Golden langur Trachypithecus geei threatened by habitat fragmentation. ZOOS' Print Journal, 17(2), 699-703. https://doi.org/10.11609/JoTT.ZPJ.17.2.699-703
Clevenger, A. P. & Huijser, M. P. (2011). Wildlife Crossing Structure Handbook: Design and Evaluation in North America. Publication No. FHWA-CFL/TD-11-003, U.S. Federal Highway Administration, Washington, DC.
Cunneyworth, P. M., Donaldson, A., & Onyancha, F. (2022). Canopy bridges are an economical mitigation reducing the road barrier effect for three of four species of monkeys in Diani, Kenya. Folia Primatologica, 93(3-6), 217-234.
Das, J., Biswas, J., Bhattacherjee, P. C. & Rao, S. S. (2009). Canopy Bridges: An Effective Conservation Tactic for Supporting Gibbon Populations in Forest Fragments, in S. Lappan & D. J. Whittaker (eds.), The Gibbons. Developments in Primatology: Progress and Prospects, Springer, New York, NY, pp.467–475. https://doi.org/10.1007/978-0-387-88604-6_22
Dodd, N. L., Gagnon, J. W., Boe, S., Manzo, A. & Schweinsburg, R. E. (2007). Evaluation of measures to minimize wildlife-vehicle collisions and maintain permeability across highways: Arizona Route 260. Final Report, 540. Arizona Game and Fish Department, Research Branch, Arizona, USA.
Donaldson, A. & Cunneyworth, P. (2015). Case study: canopy bridges for primate conservation, in R. van der Ree, D. J. Smith & C. Grilo (eds.), Handbook of Road Ecology, John Wiley & Sons, West Sussex, UK, pp.341–343. https://doi.org/10.1002/9781118568170.ch41
Frankham, R., Ballou, J. D. & Briscoe, D. A. (2004). A Primer of Conservation Genetics, Cambridge University Press, p.236. https://doi.org/10.1017/CBO9780511817359
Jeganathan, P., Mudappa, D., Raman, T. S. & Kumar, M. A. (2018). Understanding perceptions of people towards lion-tailed macaques in a fragmented landscape of the Anamalai Hills, Western Ghats, India. Primate Conservation, 32(11), 205-215.
Lokschin, L. X., Printes, R. C., Cabral, J. N. H. & Buss, G. (2007). Power lines and howler monkey conservation in Porto Alegre, Rio Grande do Sul, Brazil, Neotropical Primates, 14(2), 76–80. https://doi.org/10.1896/044.014.0206
Maria, M., Al-Razi, H., Ali, A., Muzaffar, S. B. & Nekaris, K. A. I. (2022). Artificialcanopy bridge use by primates and other arboreal mammals in a fragmented tropical forest of northeast Bangladesh, Folia Primatologica, 93(3-6), 217–234. https://doi.org/10.1163/14219980-20211201
Mass, V., Rakotomanga, B., Rakotondratsimba, G., Razafindramisa, S., Andrianaivomahefa, P., Dickinson, S. & Cooke, A. (2011). Lemur bridges provide crossing structures over roads within a forested mining concession near Moramanga, Toamasina Province, Madagascar, Conservation Evidence, 8, 11–18.
Medhi, R., Chetry, D., Bhattacharjee, P. C. & Patiri, B. N. (2004). Status of Trachypithecus geei in a rubber plantation in Western Assam, India, International Journal of Primatology, 25(6), 1331–1337. https://doi.org/10.1023/B:IJOP.0000043965.38722.63
Onderdonk, D. A. & Chapman, C. A. (2000). Coping with forest fragmentation: the primates of Kibale National Park, Uganda, International Journal of Primatology, 21(4), 587–611. https://doi.org/10.1023/A:1005509119693
Praill, L. C., Eppley, T. M., Shanee, S., Cunneyworth, P. M. K., Abra, F. D., Allgas, N., Al-Razi, H., Campera, M., Cheyne, S. M. et al. (2023). Road Infrastructure and Primate Conservation: Introducing the Global Primate Roadkill Database, Animals, 13(10), 1692. https://doi.org/10.3390/ani13101692
Python Software Foundation (2025). Python (Version 3.13). Available at: https://www.python.org
QGIS Development Team (2025). QGIS Geographic Information System (Version 3.42). https://qgis.org
Ram, M. S., Kittur, S. M., Biswas, J., Nag, S., Shil, J. & Umapathy, G. (2016). Genetic diversity and structure among isolated populations of the endangered Gee’s golden langur in Assam, India, PLoS One, 11(8), e0161866. https://doi.org/10.1371/journal.pone.0161866
Shil, J., Biswas, J. & Kumara, H. N. (2020). Influence of habitat conditions on group size, social organization, and birth pattern of golden langur (Trachypithecus geei), Primates, 61(6), 797–806. https://doi.org/10.1007/s10329-020-00829-y
Shil, J., Biswas, J., Nag, S. & Kumara, H. N. (2021). Persistence of Trachypithecus geei (Mammalia: Primates: Cercopithecidae) in a rubber garden in Assam, India, Journal of Threatened Taxa, 13(7), 18679–18686. https://doi.org/10.11609/jott.7273.13.7.18679-18686
Soanes, K., Goldingay, R. & van der Ree, R. (2013). Movement re-established but not restored: inferring the effectiveness of built crossing structures for gliding mammals, Biological Conservation, 159, 86–93. https://doi.org/10.1016/j.biocon.2012.10.016
Soanes, K., Rytwinski, T., Fahrig, L., Huijser, M. P., Jaeger, J. A. G., Teixeira, F. Z., van der Ree, R. & van der Grift, E. A. (2024). Do wildlife crossing structures mitigate the barrier effect of roads on animal movement? A global assessment, Journal of Applied Ecology, 61(2), 271–289. https://doi.org/10.1111/1365-2664.14582
Soanes, K., Taylor, A. C., Sunnucks, P., Vesk, P. A., Cesarini, S. & van der Ree, R. (2018). Evaluating the success of wildlife crossing structures using genetic approaches and an experimental design: lessons from a gliding mammal, Journal of Applied Ecology, 55(1), 129–138. https://doi.org/10.1111/1365-2664.12966
Srivastava, A., Biswas, J., Das, J. & Bujarbarua, P. (2001). Status and distribution of golden langurs (Trachypithecus geei) in Assam, India, American Journal of Primatology, 55(1), 15–23. https://doi.org/10.1002/ajp.1035
Thinley, P., Norbu, T., Rajaratnam, R., Vernes, K., Wangchuk, K., Choki, K., Tenzin, J., Tenzin, S. & Kinley et al. (2019). Population abundance and distribution of the endangered golden langur (Trachypithecus geei, Khajuria 1956) in Bhutan, Primates, 60(5), 437-448. https://doi.org/10.1007/s10329-019-00737-w
van der Grift, E. A. & van der Ree, R. (2015). Guidelines for evaluating use of wildlife crossing structures, in R. van der Ree, D. J. Smith & C. Grilo (eds.), Handbook of Road Ecology, John Wiley & Sons, Chichester, 411–419. https://doi.org/10.1002/9781118568170.ch15




